NEWSADVISORIES and ANNOUNCEMENTSCOVID-19 Resources#DTIStories

ORLANDO, FLORIDA -- The Philippines’ 7th participation in the HIMSS Global Conference and Exhibition (HIMSS24) generated an initial sales amounting to US$ 53 million (inclusive of closed deals, contracts under negotiation, potential sales to be nurtured) plus additional potential direct investment amounting to US$ 50 million in the next three
Read more. MAKATI CITY–On April 17, the Department of Trade and Industry (DTI) signed a memorandum of understanding (MOU) with Jollibee Foods Corporation (JFC) and Jollibee Group Foundation (JGF), aimed at the empowerment of micro, small, and medium enterprises (MSMEs). This sealed partnership aligns with DTI Secretary Fred Pascual's strategic vision for
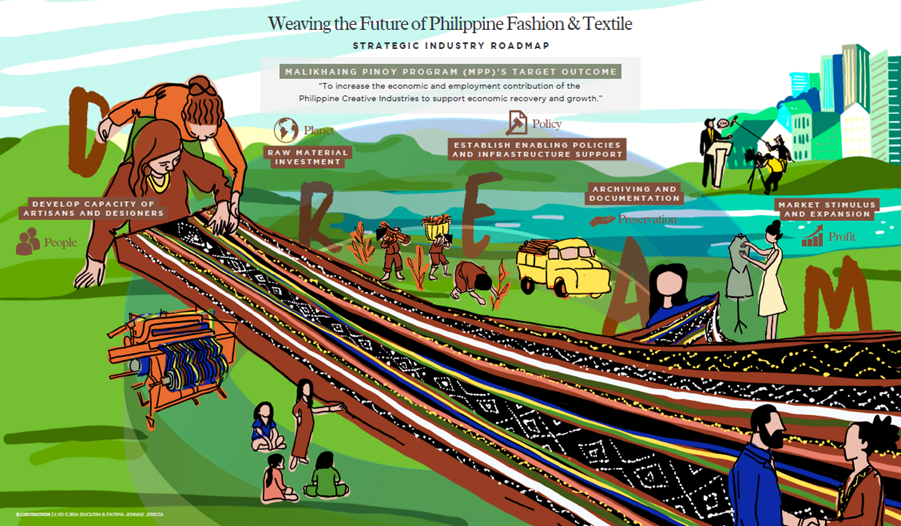
Read more. MAKATI CITY -- The Department of Trade and Industry (DTI), through its Bureau of Industry Planning and Innovation (BIPI), successfully conducted a validation session for the results of its fashion and textile industries roadmap project on 16 April 2024 at Valero Grand Suites, Makati City. As part of BIPI’s Creative
Read more.Please click here if you are not redirected within a few seconds.
Featured Success Stories
A tale of vision, resilience, and unwavering commitment to the Pinoy legacy. A diamond in the rough Amid the idyllic backdrop of Davao del Norte's hinterlands, a tale of chocolate unfolds. In a time when roads remained unpaved, and the journey from bustling Davao City to the tranquil haven of
Read more. GameOps Inc. is part of the gaming future, at home and abroad. A summit of Innovation In February 2024, the picturesque shores of Boracay—a small island in central Philippines—became the epicenter of the gaming world with the GameDev Summit. Amidst this confluence of creativity and tech, GameOps Inc. emerged as
Read more.Zero to Hero
In the Philippines, calamansi juice is a staple beverage. It is refreshing and flavorful, and it’s packed with Vitamin C — making it a great choice for boosting your immune system. It’s the kind of thing that would be in demand during a health crisis. Owing to its popularity and
Read more. Who would’ve thought that a graduation project would become an opportunity to shed light on someone else’s life? Gleizl Joy Soo, also known as Joy to her friends and peers, combined her love for fashion and her search for her life’s purpose in her small enterprise, Musa Fabric. From Fashion
Read more.News Features
The Philippines' history is marked by its strategic geographic position, which, prior to its dynamic and multifaceted trade relationships with global partners, attracted European interest for its pivotal role as an entry point to the riches of Asia. This interest, spanning from several centuries of Spanish influence to a brief
Read more. Do you still remember the first time you smelled the oozing aroma of coffee? The mixture of bittersweet yet comforting feelings that probably gets you excited every time you wake up or take a break from your usual 8 to 5 routine. The familiarity that lingers ever so delicately, you
Read more.